ABOUT BREPOCITINIB
Priovant’s lead molecule is brepocitinib, an oral once-daily dual inhibitor of TYK2 and JAK1. By selectively inhibiting both TYK2 and JAK1, brepocitinib is optimized to suppress signaling of a wide range of TYK2- and JAK1-dependent cytokines linked to autoimmunity, including type I and type II interferon, IL-6, IL-12, and IL-23.
Brepocitinib has been evaluated in seven successful Phase 2 studies that have generated clinically meaningful efficacy results. Brepocitinib’s safety database includes over 1,400 exposed subjects and patients and suggests a safety profile similar to those of approved JAK inhibitors.
Priovant is developing brepocitinib across multiple severe autoimmune diseases with few approved therapies and pathobiologies aligned with the distinctive benefits of dually inhibiting both TYK2 and JAK1. Priovant is currently running the VALOR Study, a single registrational Phase 3 trial evaluating brepocitinib in dermatomyositis patients, with top-line results expected in 2025. Priovant is also expecting to initiate a Phase 3 program in non-infectious uveitis (NIU) in the second half of 2024, following successful results from the Phase 2 NEPTUNE Study.
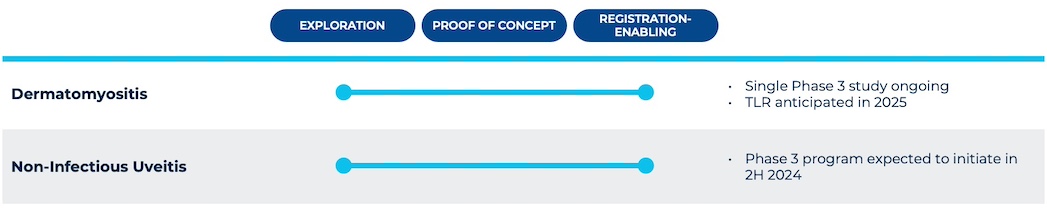
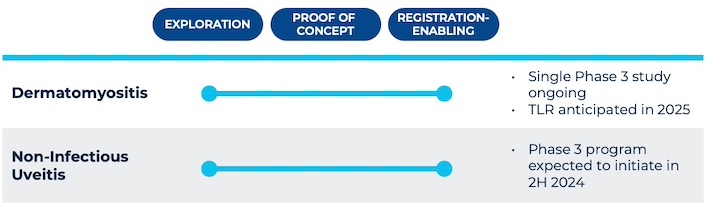
Priovant is also exploring additional potential indications with high unmet need and for which dual inhibition of JAK1 and TYK2 is expected to provide differentiated benefit.
ABOUT DERMATOMYOSITIS
Dermatomyositis is an immune-mediated disease of the skin and muscles. Patients with dermatomyositis usually present with a characteristic skin rash and debilitating muscle weakness, which may lead to significant functional impairment and/or disfigurement. Substantially increased risk of interstitial lung disease, malignancy, and heart failure contribute to an estimated five-year mortality rate of 10-40%. Existing dermatomyositis therapies are associated with serious safety risks and burdensome administration, and many patients will not respond adequately. There is accordingly a substantial unmet need for novel, efficacious and convenient therapies that address the underlying pathophysiology of dermatomyositis.
Priovant is currently running the VALOR Study to evaluate the use of brepocitinib in dermatomyositis patients. Clinical evidence from a previous investigator-initiated study of tofacitinib, an approved JAK1/2/3 inhibitor, and over 100 off-label case reports suggests that JAK1 inhibition is efficacious in dermatomyositis. Since dermatomyositis pathobiology is driven by dysregulations in cytokines whose signaling is mediated by both TYK2 and JAK1, there may be potential for greater efficacy for brepocitinib compared to tofacitinib, selective JAK1 inhibitors, and selective TYK2 inhibitors.
ABOUT NON-INFECTIOUS UVEITIS
Non-infectious uveitis (NIU) is a vision-threatening ocular autoimmune disease. NIU is the fourth-leading cause of blindness among the working age population in the developed world and is responsible for 10% of cases of blindness in the United States. There is only one approved non-steroidal therapy for NIU, which has limited efficacy, with more than 50% of patients failing to achieve disease remission. Even among patients whose ocular inflammation is effectively managed, serious sequelae of NIU, like macular edema, remain a major challenge for patients and physicians.
Priovant recently announced positive topline results from the Phase 2 NEPTUNE study evaluating brepocitinib in patients with active NIU. In this study, Brepocitinib 45 mg once daily achieved the best observed efficacy results on the NIU registrational endpoint of any active NIU clinical trial conducted to-date (more details are available in the Priovant Overview Deck). Additionally, brepocitinib demonstrated proof-of-concept for potentially preventing and treating uveitic macular edema, a key long-term cause of vision loss in patients with NIU. Priovant expects to launch a Phase 3 clinical program in NIU in 2H 2024.